
COVID-19 Vaccines for Kids Under 5 Receive Emergency Use Authorization
It’s been 1 ½ years since the first COVID-19 vaccine received an emergency use authorization through the Food and Drug Administration (FDA). Since then, many parents of young children have been waiting for the opportunity to protect them from COVID-19. Having a vaccine available for children 5-17 years old was an exciting step, and now another layer of protection for children 6 months – 4 years has received emergency use authorization through the FDA, which was then reviewed and approved by both the Center for Disease Control and Prevention and the Western States Safety Review Board. With each approval there are always questions, so let’s dig into questions that are coming up right now.

Why did it take so much longer for this age group?
When the world began working on a vaccine for COVID-19, the priority group was adults because it was clear this group was the most at risk of hospitalization and death. Through amazing collaboration and the world coming together to provide resources for this vaccine, scientists were able to efficiently create a vaccine for the most vulnerable. Scientists then quickly began working on a vaccine for children knowing there was still a high disease burden throughout all age groups.
Why does this age group need vaccinated when there is a lower risk than adults?
Just because children have had less risk associated with COVID-19 doesn’t mean they have no risk. If you look at documents from the Vaccines and Related Biological Products Advisory Committee Meeting, you can see that just because they don’t have the same risk as adults, their risk is still great. This information is not to scare parents, but it is needed to make an informed decision about whether there is more risk in vaccinating or not vaccinating your child.
Infants and Young Children Do Die from COVID-19
Deaths involving COVID-19 in US Children 0-4 Years
422 total deaths 2020-2022 (as of June 2)
- 74 Deaths in 2020
- 221 Deaths in 2021
- 147 Deaths in 2022 (as of June 2)
As you can see below, COVID-19 also has much worse outcomes than many of the other diseases we vaccinate our children from.
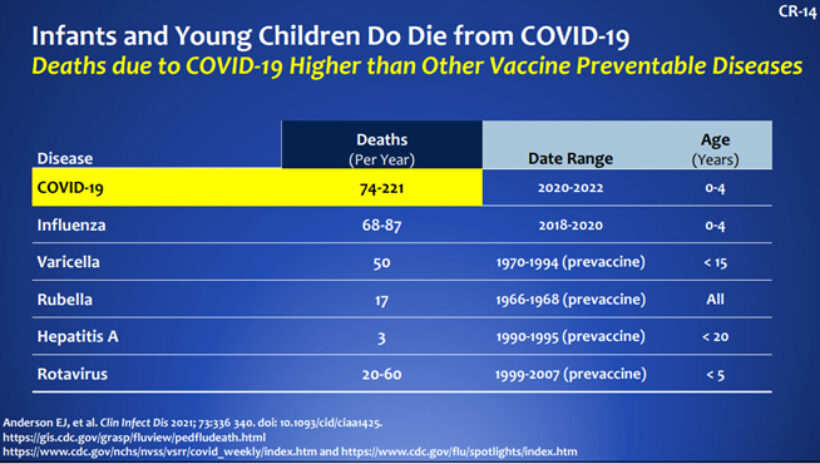
CDC.gov
We need to make sure we do not become numb to the risk for children just because it seems so much less than in adults. The best information to evaluate when asking if you should vaccinate your child is the comparison of risk between vaccinated and unvaccinated children in their age group, not the comparison of risk associated with age.
What if I’m already getting anxious about the decision?
We get it! This decision can feel overwhelming. We hear a lot from both sides, but the reality is most parents are somewhere in the middle trying to decide what is best for their child. You can check out our previous blog about ways to help you through the decision process.
How is our area preparing to distribute this new round of vaccines?
This round of vaccines will be a different than previous distributions. The main reason is that the children are a lot younger and not all providers feel comfortable giving immunizations to that age. That’s why we love our pediatricians! They know how to help the kids (and the parents) have the best experience possible when get a vaccine.
SRHD will help as many providers as possible order the vaccine by being a vaccine depot. Vaccine depots are locations that store vaccine for their area. Providers can order vaccine from a depot in smaller amounts than they can through the Washington State Immunization Information System. This opportunity allows providers to administer COVID-19 vaccines who otherwise wouldn’t be able to.
If you’re relieved and ready to have your child vaccinated, make an appointment with their pediatrician to discuss options and what is best for your child. If you’re nervous and unsure about having your child vaccinated, we also encourage you to make an appointment with their pediatrician. They are ready to answer your questions, address your concerns, and help you understand what is best for your child based on their health history.
